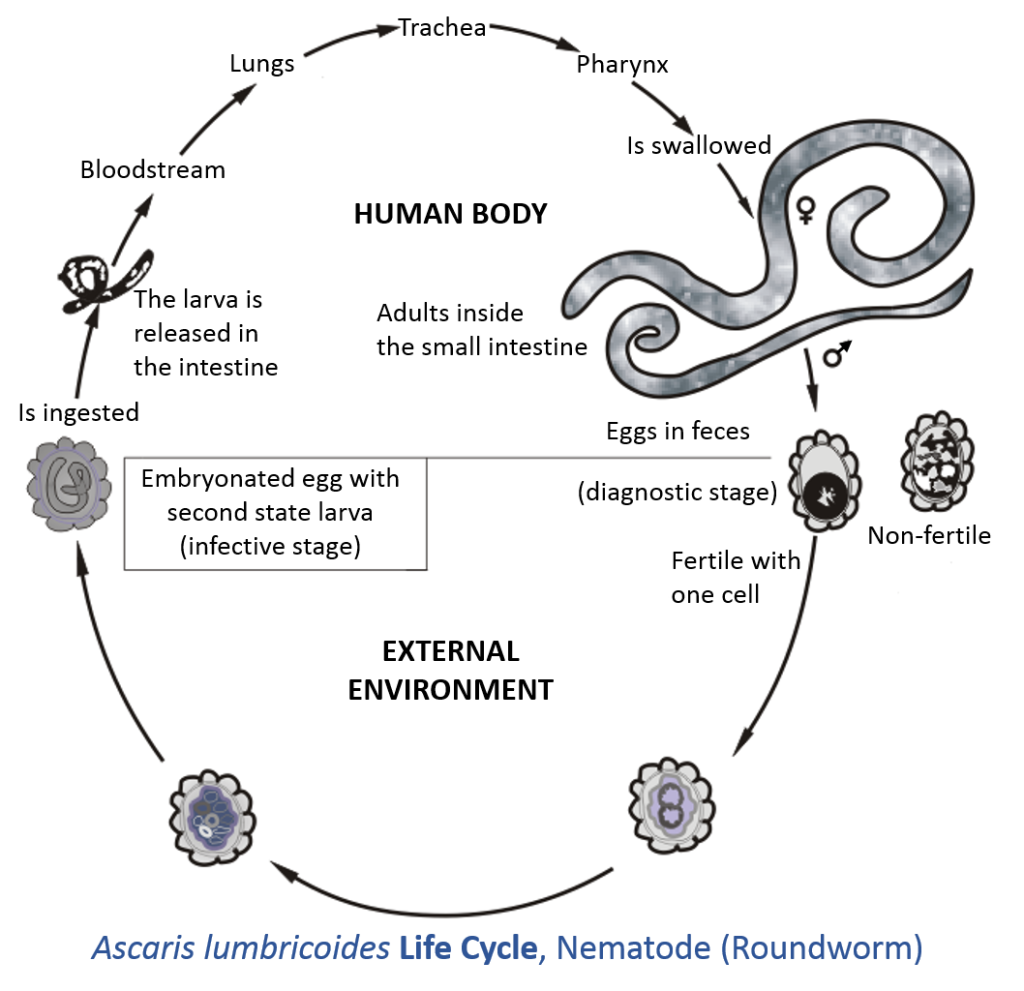
Have you made a New Year’s resolution to read #260papers or #365papers this year? Or maybe you want to do some relaxing reading during your holiday break? I’ve got you covered! Last April, I posted some of the great parasite ecology papers I’d seen in 2020. This is another list of 2020 papers that you might like. Here’s to hoping that 2021 has more great parasite ecology papers and fewer pandemics. Happy reading and Happy New Year!
Albery, G.F., Kirkpatrick, L., Firth, J.A. & Bansal, S. (2020). Unifying spatial and social network analysis in disease ecology. J. Anim. Ecol.
Aleuy, O.A. & Kutz, S. (2020). Adaptations, life-history traits and ecological mechanisms of parasites to survive extremes and environmental unpredictability in the face of climate change. Int. J. Parasitol.-Parasit. Wildl., 12, 308–317.
Bailes, E.J., Bagi, J., Coltman, J., Fountain, M.T., Wilfert, L. & Brown, M.J.F. (2020). Host density drives viral, but not trypanosome, transmission in a key pollinator. Proc. R. Soc. B-Biol. Sci., 287, 20191969.
Baines, C.B., Diab, S. & McCauley, S.J. (2020). Parasitism Risk and Infection Alter Host Dispersal. Am. Nat., 196, 119–131.
Barnett, K.M. & Civitello, D.J. (2020). Ecological and Evolutionary Challenges for Wildlife Vaccination. Trends Parasitol., 36, 970–978.
Bienentreu, J.-F. & Lesbarreres, D. (2020). Amphibian Disease Ecology: Are We Just Scratching the Surface? Herpetologica, 76, 153–166.
Carlson, C.J., Dallas, T.A., Alexander, L.W., Phelan, A.L. & Phillips, A.J. (2020). What would it take to describe the global diversity of parasites? Proceedings of the Royal Society B: Biological Sciences, 287, 20201841.
Ellner, S.P., Ng, W.H. & Myers, C.R. (2020). Individual Specialization and Multihost Epidemics: Disease Spread in Plant-Pollinator Networks. Am. Nat., 195, E118–E131.
Espinola-Novelo, J.F., Teresa Gonzalez, M., Pacheco, A.S., Luque, J.L. & Oliva, M.E. (2020). Testing for deterministic succession in metazoan parasite communities of marine fish. Ecol. Lett., 23, 631–641.
Hafer-Hahmann, N. & Vorburger, C. (2020). Parasitoids as drivers of symbiont diversity in an insect host. Ecol. Lett., 23, 1232–1241.
Halliday, F.W., Heckman, R.W., Wilfahrt, P.A. & Mitchell, C.E. (2020a). Eutrophication, biodiversity loss, and species invasions modify the relationship between host and parasite richness during host community assembly. Glob. Change Biol., 26, 4854–4867.
Halliday, F.W., Rohr, J.R. & Laine, A.-L. (2020b). Biodiversity loss underlies the dilution effect of biodiversity. Ecol. Lett., 23, 1611–1622.
Lohr, J.N. & Haag, C.R. (2020). Parasite-driven replacement of a sexual by a closely related asexual taxon in nature. Ecology, 101, e03105.
Maestri, R., Fiedler, M.S., Shenbrot, G.I., Surkova, E.N., Medvedev, S.G., Khokhlova, I.S., et al. (2020). Harrison’s rule scales up to entire parasite assemblages but is determined by environmental factors. J. Anim. Ecol., 89, 2888–2895.
Marien, J., Borremans, B., Verhaeren, C., Kirkpatrick, L., Gryseels, S., Gouey de Bellocq, J., et al. (2020). Density dependence and persistence of Morogoro arenavirus transmission in a fluctuating population of its reservoir host. J. Anim. Ecol., 89, 506–518.
Mcdonald, R.A., Wilson-Aggarwal, J.K., Swan, G.J.F., Goodwin, C.E.D., Moundai, T., Sankara, D., et al. (2020). Ecology of domestic dogs Canis familiaris as an emerging reservoir of Guinea worm Dracunculus medinensis infection. Plos Neglect. Trop. Dis., 14, e0008170.
Payne, E., Sinn, D.L., Spiegel, O., Leu, S.T., Wohlfeil, C., Godfrey, S.S., et al. (2020). Consistent individual differences in ecto-parasitism of a long-lived lizard host. Oikos, 129, 1061–1071.
Penk, S.R., Bodner, K., Soto, J.S.V., Chenery, E.S., Nascou, A. & Molnar, P.K. (2020). Mechanistic models can reveal infection pathways from prevalence data: the mysterious case of polar bears Ursus maritimus and Trichinella nativa. Oikos.
Sauer, E.L., Cohen, J.M., Lajeunesse, M.J., McMahon, T.A., Civitello, D.J., Knutie, S.A., et al. (2020). A meta-analysis reveals temperature, dose, life stage, and taxonomy influence host susceptibility to a fungal parasite. Ecology, 101, e02979.
Timi, J.T. & Poulin, R. (2020). Why ignoring parasites in fish ecology is a mistake. Int. J. Parasit., 50, 755–761.
Weiss, M.N., Franks, D.W., Balcomb, K.C., Ellifrit, D.K., Silk, M.J., Cant, M.A., et al. (2020). Modelling cetacean morbillivirus outbreaks in an endangered killer whale population. Biol. Conserv., 242, 108398.