Ecologists sometimes use the terms “mutualism” and “symbiosis” interchangeably, and I wish that they would not do so! I recently bought a copy of Judie Bronstein’s new book about mutualism, and the first two chapters are devoted to defining the terms mutualism and symbiosis, and distinguishing between the two. Here, I’ll outline the main points. For a longer treatment of this topic, check out the book!
Mutualism is an ecological interaction between at least two species (=partners) where both partners benefit from the relationship.
Symbiosis is an ecological interaction between at least two species (=partners) where there is persistent contact between the partners.
In symbioses, one partner is often smaller, lives on or in the larger partner, and has a shorter lifespan that the larger partner. The smaller partner is a symbiont, and the larger partner is a host.
Not all mutualisms are symbioses. Some mutualisms are non-persistent, like when a pollinator very briefly visits a flower and then never returns to that flower again. Additionally, not all symbiosis are mutualistic. For instance, parasites are also symbionts.
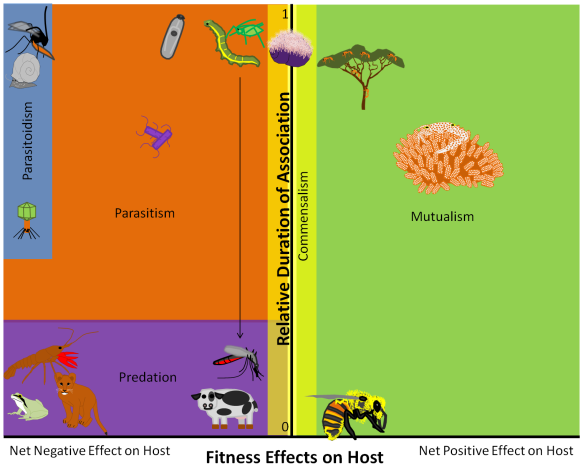
A few months ago, I posted this useful classification scheme for thinking about different types of ecological interactions. The two axes are the “relative duration of association” and “net effect of the relationship on the ‘host.’” Mutualisms are any relationships on the right-hand/positive side of the net effect of the relationship on the ‘host’ axis, regardless of where the relationship falls on the vertical axis. Symbioses are relationships at the ‘top’ of the relative duration of association axis – where much of the symbiont’s lifespan is spent in association with the same host – regardless of where the relationship falls on the horizontal axis.