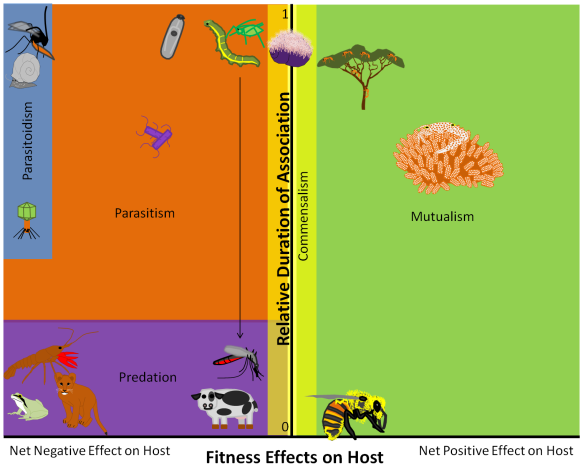
By definition, mutualists, commensals, and parasites (hereafter “affiliates” in this post) depend on their hosts for resources or services. Therefore, if a host species goes extinct, the affiliates associated with that host may go extinct, too. And in fact, coextinction events like this should be as common as – or even more common than – extinctions of hosts, because we know that every host species has many mutualists, commensals, and parasites. Just think about the mites living in your eyebrows, the bacteria living in your intestines, and that one time that you had lice in third grade. If humans disappeared, all of those affiliate species might also go extinct!
Of course, you might not believe in the intrinsic value of all species; you might be wondering why you should care if some tiny species that you’ve never heard of goes extinct. Extinctions of mutualistic species – such as the gut microbes that help you digest food and the pollinators that keep our agricultural systems running – have obvious implications for our economy and health. But parasites, too, play important roles in our lives. For instance, they regulate populations of wildlife host species, and they may prevent you from having allergic reactions to things that you shouldn’t be allergic to. And of course, species exist in intricate webs of interactions, and by accidentally (or purposely!) adding or removing species from ecosystems, we have often learned that one species can have huge impacts on ecological communities.
So, coextinctions of affiliates are important, and these coextinctions should be common. That means that we have documented tons of these coextinction events, right? Actually, we haven’t! There are very few examples of documented coextinctions (Dunn 2009, Colwell et al. 2012), and some of those are not entirely open and shut cases. But why?
Say that you document the extinction of a particular host species: Host A. Should every affiliate associated with Host A also go extinct? Because some affiliates likely use multiple host species, some of the affiliates of Host A probably survived on other host species. Also, even an affiliate that historically only used Host A might be able to continue existing if it can switch to a new host species. For instance, maybe Host B, a close relative of Host A, is a suitable alternative host.
Now imagine that you’re trying to document affiliate coextinctions as Host A disappears. What evidence might you use to figure out which affiliates have also disappeared? There might be published accounts of some of the affiliates of Host A, but there are very few host species (if any) for which every affiliate species has been documented. Therefore, the loss of one host species means that several unnamed and undescribed invertebrate species will be lost before ever being documented by humans. Even if you had a perfect list of every affiliate species, it might be really difficult to confirm whether each affiliate was now extinct. That’s because we rarely (if ever) have perfect lists of every host species used by a given affiliate species. So, if one affiliate species frequently uses three host species, but you think it is a specialist on Host A, you might think the affiliate has gone extinct, only to find it happily hanging out on Hosts B and C when you survey those species three decades later.
To summarize, we predict many coextinctions of affiliates to occur as hosts go extinct, but we have hardly documented any such coextinctions. It may be that that affiliate species are much less vulnerable than we expect due to the use of multiple host species or host species switching as a primary host goes extinct, and/or it may be that we are just very poorly equipped to observe and document these coexinctions. Clearly, if we’re going to get better estimates of affiliate coextinction rates, we need more data! Specifically, we need:
- Better understanding of the natural histories of these systems. We need complete lists of affiliates for each host species, complete lists of host species for each affiliate species, preserved specimens of affiliates for genetic identification, and information on the strengths of the interactions between each affiliate and host species.
- Better estimates of how frequently affiliates shift host species, and whether jumps to new host species are associated with declines in the availability of the current species. In other words, how often do we expect affiliates to sink with the ship versus swimming to safety? (For further reading about this, see Kiers et al. 2010.)
(It’s been too long since my last pirate worm cartoon….)

Some related reading:
Conservation – save the parasites along with the hosts?
Are pubic lice going extinct?
References:
Colwell, R.K., R.R. Dunn, N.C. Harris, and D.J. Futuyme. 2012. Coextinction and Persistence of Dependent Species in a Changing World. Annual Review of Ecology Evolution and Systematics 43: 183-203.
Dunn, R.R., N.C. Harris, R.K. Colwell, L.P. Koh, and N.S. Sodhi. 2009. The sixth mass coextinction: are most endangered species parasites and mutualists? Proc. R. Soc. B 276: 3037–3045.
Kiers, E.T., T.M. Palmer, A.R. Ives, J.F. Bruno, and J.L. Bronstein. 2010. Mutualisms in a changing world: an evolutionary perspective. Ecology Letters 13(12): 1459-1474.